Ferroalloys
Ferroalloys are master alloys containing iron and one or more nonferrous metals as alloying elements. Ferroalloys are generally divided into two categories: bulk ferroalloys (produced in large quantities in electric arc furnaces) and special ferroalloys (produced in smaller quantities but of increasing importance). Bulk ferroalloys are used exclusively in steelmaking and steel foundries, while the uses of special ferroalloys are more varied. In general, about 90% of ferroalloys are used in the steel industry.
As mentioned above, ferroalloys can be divided into two major categories: bulk alloys (
ferrochrome,
ferrosilicon, ferromanganese, silicon manganese and ferronickel) and special alloys (
ferrovanadium,
ferromolybdenum,
ferrotungsten,
ferrotitanium, ferroboron and
ferroniobium).
Production of Ferroalloys
There are two main methods of producing ferroalloys, one is the use of carbon in combination with appropriate smelting processes, and the other is metallothermic reduction with other metals. The former process is usually associated with batch operations, while the latter is mainly used to focus on specialized high-grade alloys that usually have a lower carbon content.
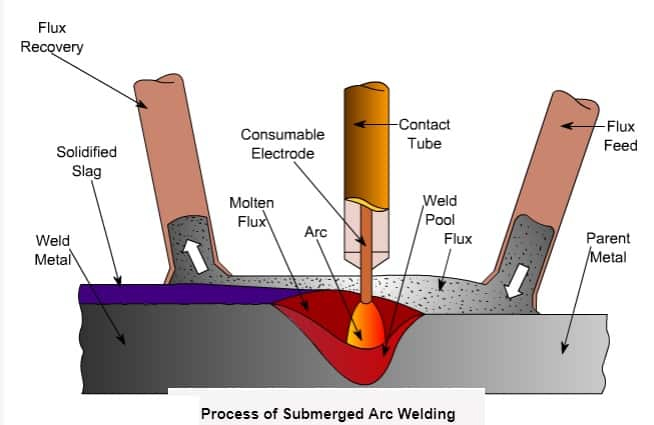
Submerged Arc Process
The submerged arc process is a reduction smelting operation. The reactants consist of metal ores (ferrous oxide, silicon oxide, manganese oxide, chrome oxide, etc.). and a reducing agent, a carbon source, usually in the form of coke, charcoal, high and low volatile coals, or sawdust. Limestone may also be added as a flux. The raw materials are crushed, graded, and in some cases, dried, before being conveyed to a mixing chamber for weighing and mixing.
Conveyors, buckets, skip elevators, or cars deliver the processed material to a hopper above the furnace. The mixture is then gravity-fed through a feed chute, either continuously or intermittently, as required. At the high temperatures of the reaction zone, the carbon source reacts with the metal oxides to form carbon monoxide and reduces the ore to base metals
Smelting in an electric arc furnace is accomplished by converting electrical energy into heat. Alternating current applied to the electrodes causes an electric current to flow through the charge between the electrode tips. This provides a reaction zone with temperatures as high as 2000°C (3632°F). As the alternating current flows between the electrode tips, the tip of each electrode continuously changes polarity. To maintain a uniform electrical load, the electrode depth is automatically varied continuously by mechanical or hydraulic means.
Exothermic (metallothermic) processes
Exothermic processes are commonly used to produce high-grade alloys with low carbon content. The intermediate molten alloy used in this process can come directly from a submerged arc furnace or from another type of heating device. Silicon or aluminum combines with oxygen in the molten alloy, resulting in a sharp rise in temperature and intense stirring of the molten bath.
Ferrochromium (FeCr) and ferromanganese (FeMn) of low and medium carbon content are produced by silicon reduction. Aluminum reduction is used to produce metallic chromium,
ferrotitanium,
ferrovanadium and ferroniobium.
Ferromolybdenum and
ferrotungsten are produced by a mixed aluminum and silicon heat treatment process. Although aluminum is more expensive than carbon or silicon, the product is purer. Low carbon (LC) ferrochromium is usually produced by melting chrome ore and lime in a furnace.
A specified amount of the molten ferrosilicon is then placed in a steel ladle. A known amount of intermediate grade ferrosilicon is then added to the ladle. The reaction is extremely exothermic and liberates chromium from its ore, producing LC ferrochrome and calcium silicate slag. This slag, which still contains recoverable chromium oxide, reacts with the molten high carbon ferrochrome in a second ladle to produce medium grade ferrochrome. Exothermic processes are usually carried out in open vessels and may produce emissions similar to submerged arc processes for a short period of time during the reduction process.
.jpg)